Don't worry about unexpected IRB review fees.
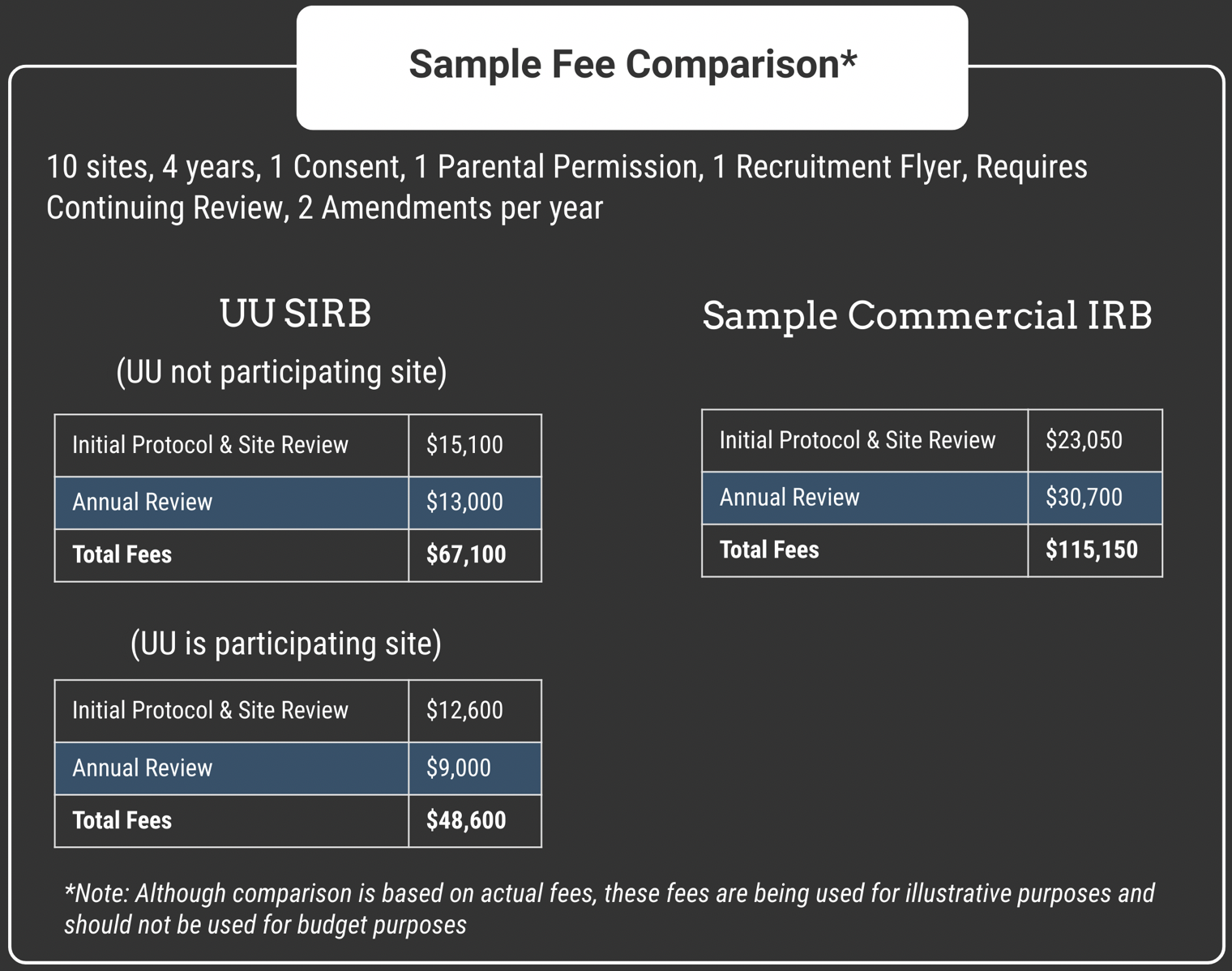
The UU SIRB follows a flat rate fee structure for all federally-funded projects. We provide a fee quote to be included in your federal grant budget based on the number of sites and length of project. If those factors remain the same, you will only pay the fees that were part of the quote - no matter how many changes/modifications/amendments you might need.
There will be an initial review fee for the protocol and each site, and annual fee for earch site. The annual fee includes all amendments, documents, and renewal/continuing review submissions

Competitive Rates
The University of Utah IRB is a department within the University of Utah and not a for-profit entity. Founded in 1850, the University of Utah is the state's public flagship institution and top-tier research university.
UU IRB acts independently from the University with its approval and review of research, there is no pressure or potential conflict with a board of directors or venture capital funding allowing for competitive review fee rates.

UU Fee Waiver
As a major research institution, the University of Utah is a recipient of federal research grants. In order to maintain compliance with federal guidelines, when the university of Utah is a research site in your study (including data coordinating services), the UU SIRB will waive the portions of your single IRB review fees related to protocol review.
For more information, please contact us for a consultation and fee quote.

Compliance
By utilizing a flat rate fee model, all submissions are included in the annual fee.
By not allowing cost to become a factor when it comes to submitting required changes, investigators can focus on updating information or protocols when needed, reducing the potential for deviations.
There is never a charge for review of an event report or deviation report.

Consent Forms
Some IRBs charge additional fees if a study has more than one consent form. The UU SIRB's fee structure means there is no additional charge for multiple consent documents.
A study should have as many consent documents as needed based on the protocol, not costs. If it's best for participants to have different consent forms or multiple translations, do what's best for participants, not your budget.